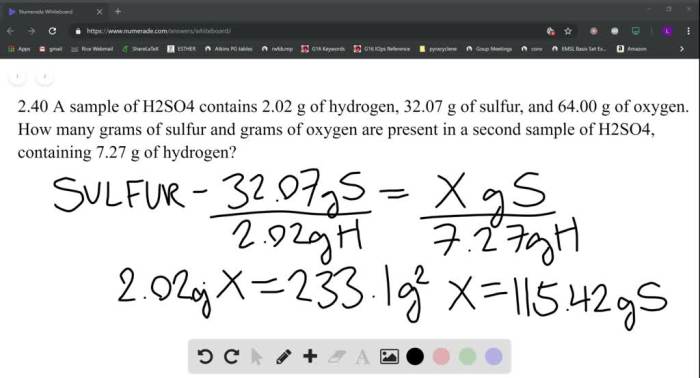
For each solute click the button under the better solvent – In the realm of chemistry, solvent selection plays a pivotal role in determining the effectiveness of various chemical processes. This article delves into the intricacies of solvent properties, guiding readers through the process of selecting the optimal solvent for a given solute.
By understanding the factors that influence solvent effectiveness, we can optimize chemical reactions, improve product yields, and enhance the efficiency of scientific research.
The concept of “like dissolves like” serves as a fundamental principle in solvent selection. Polar solvents, characterized by their ability to form hydrogen bonds, are best suited for dissolving polar solutes. Conversely, nonpolar solvents are more effective in dissolving nonpolar solutes.
This principle provides a valuable starting point for selecting the appropriate solvent for a given application.
Solvent Properties: For Each Solute Click The Button Under The Better Solvent
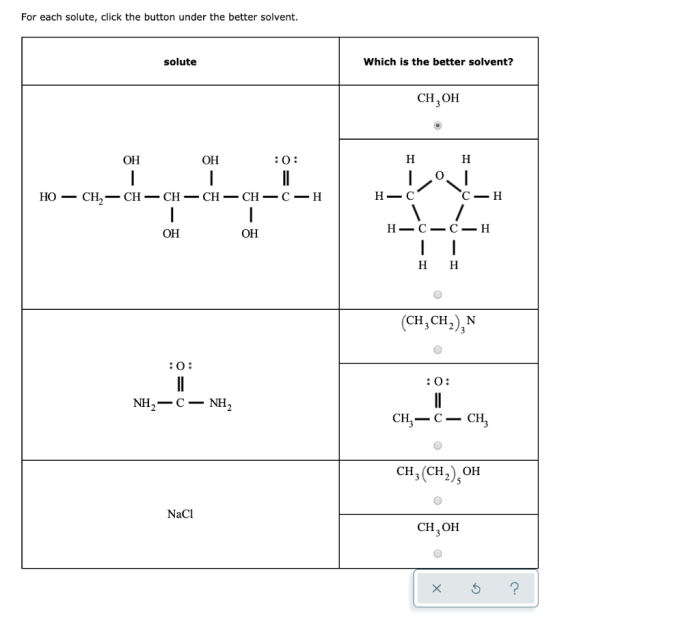
Solvents play a crucial role in various chemical processes, from extraction to purification and synthesis. The effectiveness of a solvent is determined by its ability to dissolve a solute, which depends on several factors:
- Polarity:Polar solvents, such as water and alcohols, have a partial positive or negative charge and can dissolve polar solutes.
- Hydrogen bonding:Solvents that can form hydrogen bonds, such as water and ammonia, can dissolve solutes that contain hydrogen bond donors or acceptors.
- Solubility parameters:These parameters represent the cohesive energy of a solvent and can be used to predict the solubility of solutes with similar solubility parameters.
Solvent Selection for Solutes
Selecting the best solvent for a given solute is essential for optimizing chemical processes. The concept of “like dissolves like” suggests that polar solutes dissolve best in polar solvents, while nonpolar solutes dissolve best in nonpolar solvents.
Solubility parameters provide a quantitative measure of the cohesive energy of a solvent and can be used to predict the solubility of solutes with similar solubility parameters. By matching the solubility parameters of the solvent and solute, the best solvent can be selected.
Experimental Procedures
To determine the best solvent for a solute, an experiment can be designed involving the following steps:
- Select a range of solvents with different polarities and solubility parameters.
- Add a known amount of solute to each solvent.
- Stir or shake the solutions until the solute is completely dissolved or reaches equilibrium.
- Record the amount of solute that dissolves in each solvent.
The solvent that dissolves the most solute is considered the best solvent for that particular solute.
Data Analysis and Interpretation
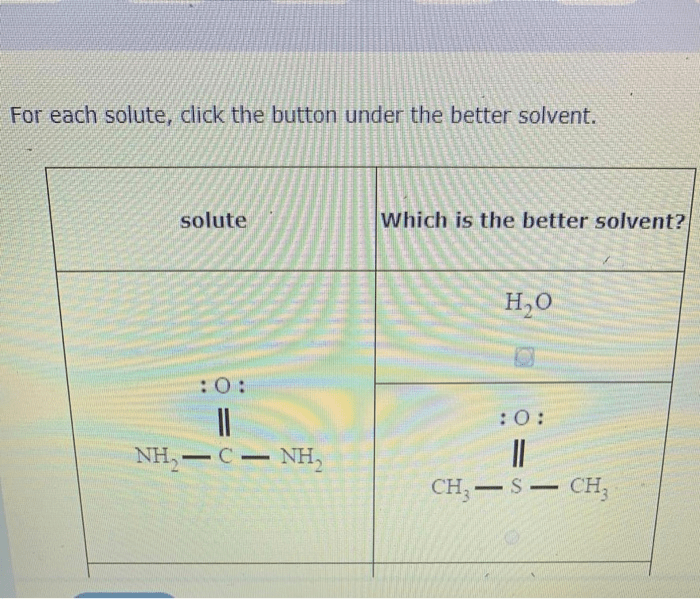
The experimental results can be analyzed by plotting the amount of solute dissolved against the polarity or solubility parameters of the solvents. The solvent that results in the highest solubility is the best solvent for the solute.
Factors that may influence the results include the temperature, pressure, and presence of other solutes in the solution.
Applications of Solvent Selection
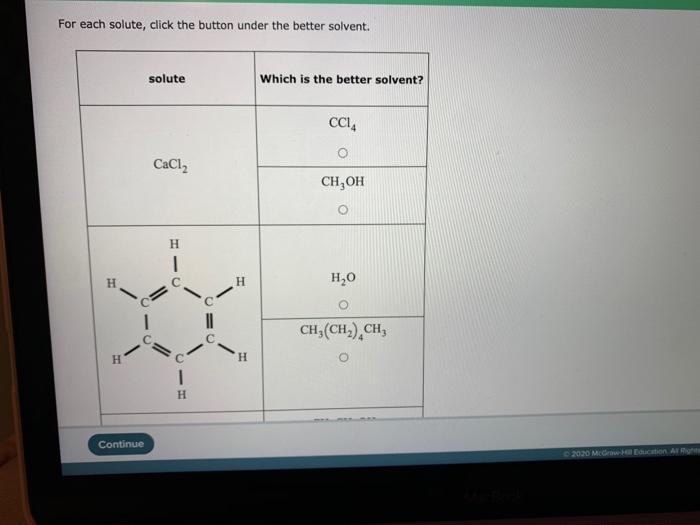
Solvent selection has numerous applications in various fields, including:
- Chemistry:Selecting the best solvent for a reaction can improve the yield and selectivity of the reaction.
- Pharmacology:Choosing the right solvent for drug delivery can enhance bioavailability and reduce side effects.
- Environmental protection:Selecting environmentally friendly solvents can minimize the impact on the environment.
Detailed FAQs
What is the most important factor to consider when selecting a solvent?
The polarity of the solvent and the solute is the most important factor to consider when selecting a solvent.
What is the concept of “like dissolves like”?
The concept of “like dissolves like” states that polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
What are some common applications of solvent selection?
Solvent selection is used in a wide range of applications, including chemical synthesis, extraction, purification, and chromatography.