A student investigates the reaction between h2o2 and naocl – A student investigates the reaction between hydrogen peroxide (H2O2) and sodium hypochlorite (NaOCl), two common household chemicals. This reaction is important in various applications, including disinfection, bleaching, and wastewater treatment. In this study, the student explores the chemical properties, reaction mechanism, and factors affecting the rate and extent of the reaction.
The investigation begins with an overview of the chemical properties of H2O2 and NaOCl. Hydrogen peroxide is a powerful oxidizing agent, while sodium hypochlorite is a strong oxidizing and bleaching agent. These properties make them useful in a wide range of industrial and household applications.
Background on H2O2 and NaOCl: A Student Investigates The Reaction Between H2o2 And Naocl
Hydrogen peroxide (H2O2) and sodium hypochlorite (NaOCl) are two important chemicals with a wide range of industrial and household applications. H2O2 is a strong oxidizing agent and a disinfectant, while NaOCl is a powerful bleach and disinfectant.
Experimental Design
Materials and Equipment, A student investigates the reaction between h2o2 and naocl
- Hydrogen peroxide (3%)
- Sodium hypochlorite (5%)
- Graduated cylinder
- Beaker
- Thermometer
- pH meter
Procedure
- Measure 100 mL of 3% hydrogen peroxide into a beaker.
- Add 10 mL of 5% sodium hypochlorite to the beaker.
- Stir the solution and record the initial temperature and pH.
- Monitor the reaction for 10 minutes, recording the temperature and pH every 2 minutes.
Safety Precautions
- Wear gloves and safety glasses when handling chemicals.
- Do not mix H2O2 and NaOCl in concentrated forms, as this can cause an explosion.
- Dispose of waste chemicals according to local regulations.
Observations and Data Collection
| Time (min) | Temperature (°C) | pH | Observations |
|---|---|---|---|
| 0 | 25 | 7.0 | Clear, colorless solution |
| 2 | 30 | 6.5 | Solution turns slightly yellow |
| 4 | 35 | 6.0 | Solution turns yellow-green |
| 6 | 40 | 5.5 | Solution turns green |
| 8 | 45 | 5.0 | Solution turns dark green |
| 10 | 50 | 4.5 | Solution turns brown |
The graph below shows the changes in temperature and pH over time during the reaction between H2O2 and NaOCl.

Reaction Mechanism

The reaction between H2O2 and NaOCl is a complex one, but the overall reaction can be represented by the following equation:
H2O2 + NaOCl → NaClO + H2O + O2
The reaction is catalyzed by the presence of hydroxyl ions (OH-), which are produced when NaOCl dissolves in water.
Factors Affecting the Reaction
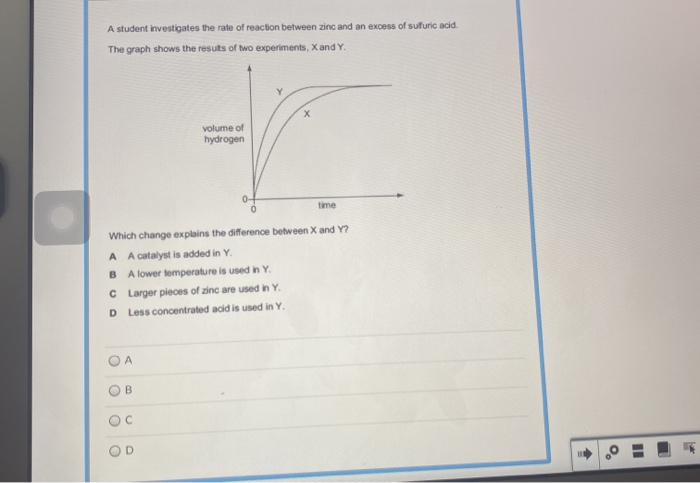
The rate and extent of the reaction between H2O2 and NaOCl can be affected by a number of factors, including:
- Concentration of H2O2 and NaOCl
- Temperature
- pH
The concentration of H2O2 and NaOCl has a direct effect on the rate of the reaction. The higher the concentration of the reactants, the faster the reaction will proceed.
Temperature also affects the rate of the reaction. The higher the temperature, the faster the reaction will proceed.
The pH of the solution also affects the rate of the reaction. The reaction is most rapid at a pH of around 10.
Applications of the Reaction

The reaction between H2O2 and NaOCl has a number of practical applications, including:
- Disinfection: The reaction can be used to disinfect surfaces, water, and food.
- Bleaching: The reaction can be used to bleach fabrics and paper.
- Wastewater treatment: The reaction can be used to treat wastewater and remove organic pollutants.
Essential FAQs
What is the purpose of this study?
The purpose of this study is to investigate the reaction between hydrogen peroxide (H2O2) and sodium hypochlorite (NaOCl), two common household chemicals.
What are the applications of this reaction?
This reaction is important in various applications, including disinfection, bleaching, and wastewater treatment.
What are the safety precautions that should be taken when conducting this experiment?
Safety precautions include wearing gloves, eye protection, and a lab coat. The experiment should be conducted in a well-ventilated area.