Welcome to the definitive guide on acids and bases, presented in an engaging and accessible format. This comprehensive acids and bases worksheet answers PDF will provide you with a thorough understanding of the fundamental concepts, reactions, applications, and implications of these crucial chemical compounds.
Acids and bases are ubiquitous in our world, playing vital roles in various chemical and biological processes. They have a profound impact on our industries, environment, and everyday lives. This worksheet delves into the fascinating realm of acids and bases, empowering you with the knowledge to navigate their complexities and appreciate their significance.
Acids and Bases Concepts
Acids and bases are two fundamental concepts in chemistry. They play a crucial role in various chemical reactions and are essential for understanding many natural phenomena.
Acids and bases have been defined in different ways throughout the history of chemistry. Some of the most important theories include:
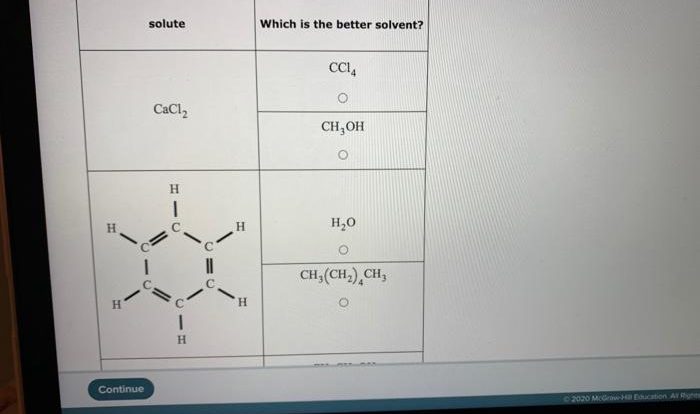
- Arrhenius theory:According to Arrhenius, an acid is a substance that produces hydrogen ions (H+) when dissolved in water, while a base is a substance that produces hydroxide ions (OH-) when dissolved in water.
- Bronsted-Lowry theory:This theory defines an acid as a substance that can donate a proton (H+), while a base is a substance that can accept a proton.
- Lewis theory:This theory defines an acid as a substance that can accept an electron pair, while a base is a substance that can donate an electron pair.
Common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
Acids and bases have distinct properties. Acids are typically sour to taste, corrosive to skin, and react with metals to produce hydrogen gas. Bases are typically bitter to taste, slippery to the touch, and react with acids to produce salts and water.
The pH of a solution is a measure of its acidity or basicity. The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic. A pH of 7 is neutral.
Acids and bases are also good conductors of electricity. The conductivity of a solution is a measure of its ability to conduct electricity. Acids and bases conduct electricity because they contain ions, which are charged particles.
Acids and bases are highly reactive substances. They can react with each other to produce salts and water. They can also react with other substances to produce a variety of products.
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+) between molecules or ions. These reactions play a crucial role in various chemical processes, including neutralization, precipitation, and gas evolution.Acid-base reactions can be classified into three main types:
Neutralization Reactions
Neutralization reactions occur between an acid and a base, resulting in the formation of a salt and water. The salt is an ionic compound composed of the cation from the base and the anion from the acid. Neutralization reactions are typically exothermic, releasing heat.For
example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O):“`HCl + NaOH → NaCl + H2O“`
Precipitation Reactions
Precipitation reactions occur between two aqueous solutions containing ions that combine to form an insoluble solid precipitate. These reactions are typically observed when the solubility product of the precipitate is exceeded.For example, the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) produces silver chloride (AgCl) as a white precipitate:“`AgNO3 + NaCl → AgCl(s) + NaNO3“`
Gas Evolution Reactions
Gas evolution reactions occur when an acid reacts with a carbonate or bicarbonate, releasing carbon dioxide (CO2) gas. These reactions are typically observed when the acid is strong and the carbonate or bicarbonate is weak.For example, the reaction between hydrochloric acid (HCl) and sodium carbonate (Na2CO3) produces carbon dioxide (CO2) gas and sodium chloride (NaCl):“`
HCl + Na2CO3 → 2NaCl + H2O + CO2(g)
“`
Acid-Base Strength
The strength of an acid or base is a measure of its ability to donate or accept protons, respectively. Strong acids and bases completely dissociate in water, releasing all their protons or hydroxide ions. Weak acids and bases only partially dissociate in water, releasing only a fraction of their protons or hydroxide ions.The
strength of an acid or base can be expressed in terms of its dissociation constant (Ka or Kb). The lower the dissociation constant, the stronger the acid or base.The strength of an acid or base affects the rate of acid-base reactions.
Strong acids and bases react quickly and completely, while weak acids and bases react slowly and incompletely.
Applications of Acid-Base Reactions, Acids and bases worksheet answers pdf
Acid-base reactions have numerous applications in various fields, including:
-
-*Neutralization
Neutralization reactions are used to neutralize acids or bases in industrial processes, environmental remediation, and medical treatments.
-*Precipitation
Precipitation reactions are used to purify solutions, remove impurities, and synthesize insoluble compounds.
-*Gas evolution
Gas evolution reactions are used to generate gases for industrial processes, such as the production of carbon dioxide for carbonated beverages.
pH and Buffers
Acids and bases are important chemical species that play a crucial role in various chemical and biological systems. pH is a measure of the acidity or alkalinity of a solution, and it is an essential factor in determining the behavior of acids and bases in these systems.
Buffers are solutions that help maintain a stable pH, making them essential for regulating pH in many biological and chemical processes.
Measuring pH
pH is measured on a scale from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while those with a pH above 7 are alkaline or basic. The pH of a solution can be measured using various methods, including pH meters, pH paper, and colorimetric indicators.
Importance of pH
pH is crucial in various chemical and biological systems. In chemical reactions, pH can affect the rate of reactions, the solubility of compounds, and the stability of molecules. In biological systems, pH plays a vital role in enzyme activity, protein structure, and cell function.
Maintaining optimal pH levels is essential for the proper functioning of living organisms.
Buffers
Buffers are solutions that resist changes in pH when small amounts of acid or base are added. Buffers consist of a weak acid and its conjugate base or a weak base and its conjugate acid. When acid is added to a buffer, the weak acid in the buffer reacts with the added acid, preventing a significant change in pH.
Similarly, when base is added, the conjugate base in the buffer reacts with the added base, again preventing a significant change in pH.
Buffers are essential for maintaining pH stability in various biological and chemical systems. For example, the human body uses buffers to regulate blood pH within a narrow range to ensure proper physiological function. Buffers are also used in industrial processes, such as food preservation and chemical manufacturing, to control pH and prevent unwanted reactions.
Applications of Acids and Bases
Acids and bases play crucial roles in various industrial, environmental, and everyday applications.
Industrial Uses
- Manufacturing:Acids are used in metal processing, electroplating, and textile production. Bases are employed in paper manufacturing, soap production, and detergents.
- Food Processing:Acids (e.g., citric acid) are used as preservatives and flavor enhancers. Bases (e.g., sodium hydroxide) are utilized in food preparation, such as making pretzels.
- Medicine:Acids and bases are essential components in pharmaceuticals, such as aspirin and antacids.
Environmental Implications
Acids and bases have significant environmental impacts:
- Acid Rain:Emissions of sulfur dioxide and nitrogen oxides from industrial activities lead to acid rain, damaging forests, lakes, and buildings.
- Water Pollution:Industrial effluents and agricultural runoff can alter water pH levels, harming aquatic ecosystems.
Everyday Applications
- Household Cleaning:Acids (e.g., vinegar) and bases (e.g., bleach) are common household cleaners.
- Personal Care Products:Acids and bases are used in shampoos, conditioners, and skincare products.
FAQ Guide: Acids And Bases Worksheet Answers Pdf
What is the difference between an acid and a base?
According to the Arrhenius theory, an acid is a substance that dissociates in water to produce hydrogen ions (H+), while a base is a substance that dissociates in water to produce hydroxide ions (OH-).
What is the pH scale?
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline (also known as “basic”). A pH of 7 is neutral.
What are some common applications of acids and bases?
Acids and bases have a wide range of applications, including in the production of fertilizers, plastics, dyes, and pharmaceuticals. They are also used in food processing, water treatment, and metalworking.